Ozone is a simple molecule made up of three oxygen atoms (O₃), yet it plays a complex and vital role in protecting life on Earth. Depending on where it is found in the atmosphere, ozone can act as a life-saving shield or a harmful pollutant. Understanding ozone helps us appreciate the balance of Earth’s climate and the need to protect this delicate layer.
What Is Ozone?
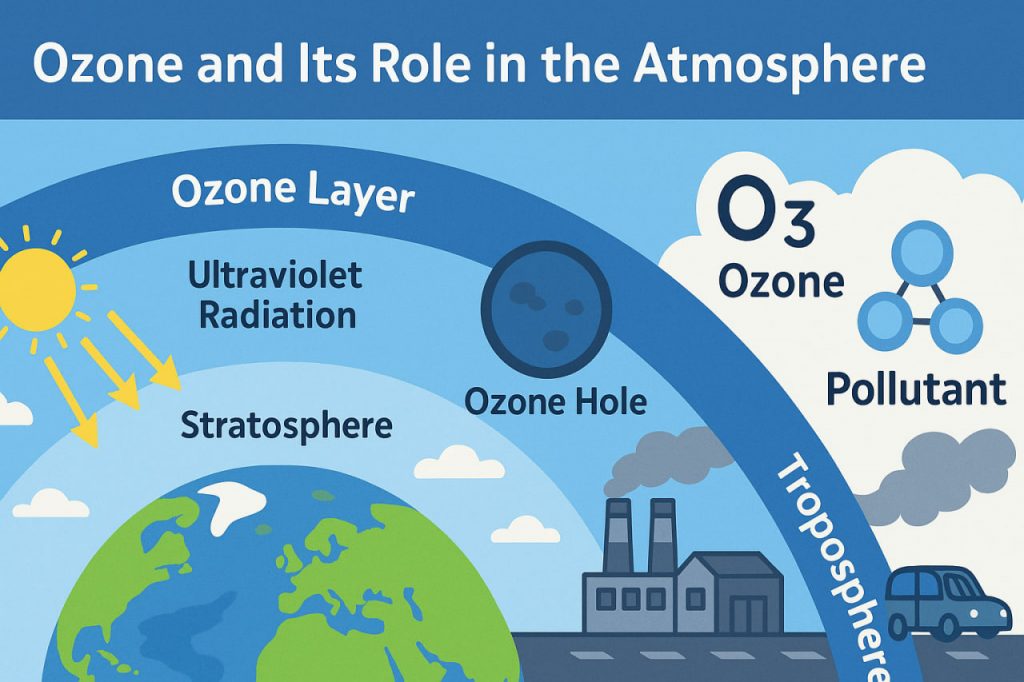
Ozone is a form of oxygen with a slightly different structure. While the oxygen we breathe (O₂) consists of two oxygen atoms, ozone has three. It naturally forms in the atmosphere when ultraviolet (UV) sunlight splits oxygen molecules and allows the free atoms to bond with other oxygen molecules.
Ozone is found in two main parts of the atmosphere:
- Stratosphere (10–50 km above Earth): Here, ozone forms the ozone layer.
- Troposphere (surface to 10 km): Here, ozone can act as a pollutant.
The Ozone Layer: Earth’s Natural Sunscreen
In the stratosphere, ozone plays a crucial role by absorbing most of the Sun’s harmful ultraviolet radiation. This ozone layer prevents excessive UV-B rays from reaching Earth’s surface, which protects living organisms from skin cancer, cataracts, and damage to plants and marine life.
Without this layer, life as we know it would struggle to survive under the intense UV exposure.
Ozone at Ground Level: A Harmful Pollutant
In the troposphere, ozone is not emitted directly but forms when sunlight reacts with pollutants such as nitrogen oxides and volatile organic compounds from car exhaust, power plants, and factories. This ground-level ozone is a major component of smog and can harm human health by irritating the lungs, especially in children, the elderly, and people with asthma.
So, while ozone is essential high in the sky, it becomes a problem when it’s too close to the ground.
The Ozone Hole and Global Response
In the 1980s, scientists discovered a thinning of the ozone layer over Antarctica, known as the ozone hole. It was caused by chlorofluorocarbons (CFCs), chemicals once used in refrigerators, air conditioners, and spray cans. These substances broke apart ozone molecules in the stratosphere.
This discovery led to the Montreal Protocol (1987), a global agreement to phase out ozone-depleting substances. Thanks to international cooperation, the ozone layer is slowly recovering, showing how science-based policy can protect the planet.
Ozone and Climate Change: Are They Related?
Although ozone depletion and climate change are separate issues, they are linked. Some ozone-depleting substances are also greenhouse gases, and changes in the ozone layer can influence weather patterns. Understanding both issues helps scientists predict how Earth’s atmosphere will respond to human activity.
Glossary
- Ozone – A molecule made of three oxygen atoms (O₃), found in both upper and lower atmosphere.
- Stratosphere – A layer of the atmosphere above the troposphere, where the ozone layer resides.
- Ultraviolet (UV) radiation – Invisible rays from the sun that can harm living tissues.
- Chlorofluorocarbons (CFCs) – Human-made chemicals that destroy ozone in the upper atmosphere.
- Montreal Protocol – An international treaty to protect the ozone layer by banning CFCs.
- Greenhouse gases – Gases that trap heat in the atmosphere, contributing to global warming.