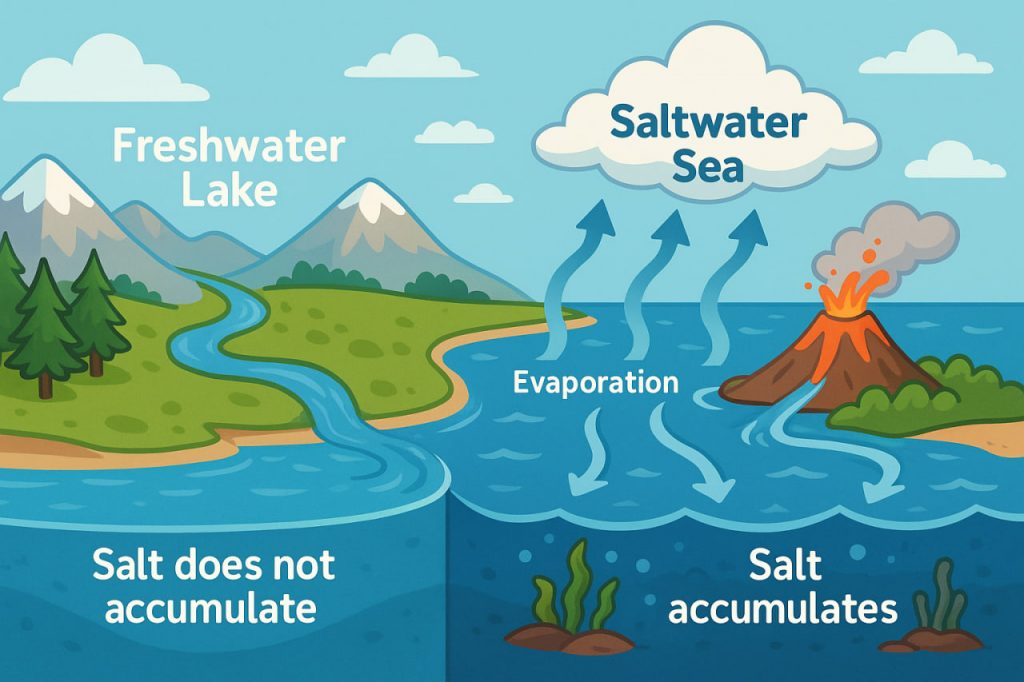
The salty taste of seawater is one of the most recognizable features of our oceans. While freshwater rivers flow constantly into the sea, carrying dissolved minerals, the oceans remain salty instead of being diluted. This is because of the balance between geological, chemical, and climatic processes that have been acting for billions of years.
1. The Origins of Ocean Salt
- When rain falls on land, it is slightly acidic due to dissolved carbon dioxide.
- This rainwater slowly erodes rocks, carrying dissolved minerals and ions (like sodium and chloride) into rivers.
- Rivers transport these dissolved substances into seas and oceans.
- Over millions of years, these salts accumulated, making oceans saline.
2. Main Components of Seawater
- Chloride (Cl⁻) and sodium (Na⁺) are the two dominant ions, together forming common table salt (NaCl).
- Other ions include magnesium, calcium, potassium, and sulfate.
- On average, ocean water contains about 3.5% salt by weight.
3. Why Oceans Stay Salty
- Water evaporates from the sea surface to form clouds, but salt does not evaporate.
- Rain returns fresh water to land, but minerals keep flowing into the ocean.
- This cycle keeps the salt in oceans constantly replenished, while rivers themselves remain mostly fresh.
4. Geological Contributions
- Volcanic eruptions release gases and minerals, adding to ocean salinity.
- Hydrothermal vents on the seafloor release minerals from Earth’s crust into seawater.
- Over time, chemical reactions remove some minerals, but the overall balance remains salty.
5. Why Not All Water Is Equally Salty
- Enclosed seas like the Dead Sea are far saltier because evaporation exceeds inflow.
- Polar regions may have less salty water due to melting ice and freshwater dilution.
- Ocean currents distribute salt unevenly, creating regional variations.
6. Importance of Salinity
- Salinity affects water density, which drives ocean currents and global climate circulation.
- Marine life has evolved to thrive in salty environments, with special adaptations for salt regulation.
- The salt in oceans also preserves Earth’s chemical balance, storing minerals for billions of years.
Conclusion
The sea is salty because of a continuous cycle of erosion, river transport, volcanic activity, and evaporation that has concentrated minerals in the oceans over geological time. Without this salinity, Earth’s oceans — and life within them — would look very different.
Glossary
- Ion – an atom or molecule with an electric charge.
- Evaporation – the process by which water turns into vapor, leaving salts behind.
- Hydrothermal vent – a fissure on the seafloor that releases hot mineral-rich water.
- Salinity – the amount of dissolved salt in water.
- Density – how heavy a substance is compared to its volume, important for ocean circulation.