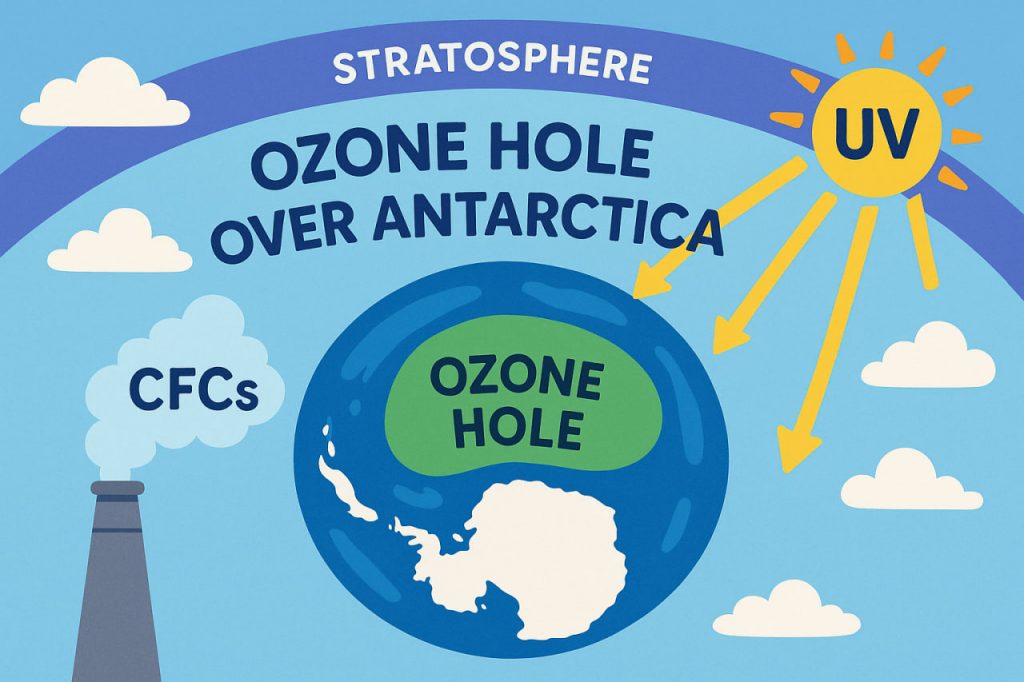
The ozone hole over Antarctica is one of the most significant environmental issues ever identified, revealing how human activity can affect the planet’s protective atmospheric layers. The ozone layer, located in the stratosphere, shields Earth from harmful ultraviolet (UV) radiation. In the late 20th century, scientists discovered that this layer was thinning dramatically over Antarctica, forming what became known as the “ozone hole.” This depletion was linked to the global use of ozone-destroying chemicals, particularly chlorofluorocarbons (CFCs). The discovery led to a worldwide effort to reduce emissions and protect atmospheric health. Although the ozone layer is slowly recovering, the Antarctic ozone hole remains a powerful reminder of the fragile balance between human industry and environmental stability.
How the Ozone Hole Formed
Ozone depletion occurs when certain industrial chemicals break apart ozone molecules (O₃) in the stratosphere. These chemicals — including CFCs, halons, and other refrigerants — rise into the upper atmosphere, where UV radiation breaks them down, releasing chlorine and bromine atoms. These atoms act as catalysts, destroying ozone much faster than it can naturally reform. The Antarctic region is particularly vulnerable because extreme cold forms polar stratospheric clouds, which accelerate ozone-destroying reactions. As atmospheric scientist Dr. Helena Vaughn explains:
“The ozone hole is not an actual hole —
it is a region of severe thinning where ozone levels drop to dangerously low values.”
This thinning allows more harmful UV radiation to reach Earth’s surface.
Why Antarctica Is Especially Affected
Antarctica’s unique combination of low temperatures, polar vortex winds, and seasonal sunlight makes it the primary site for ozone depletion. During winter, cold temperatures create ideal conditions for polar clouds. When sunlight returns in spring, chemical reactions rapidly destroy ozone. Although ozone thinning occurs globally, the Antarctic hole is the most pronounced and best studied.
Environmental and Human Health Effects
Increased UV radiation due to ozone depletion has numerous consequences:
- Higher risk of skin damage and skin cancer
- Increased cataracts and eye problems in humans and animals
- Reduced immune function
- Damage to phytoplankton, the foundation of marine food webs
- Harm to plant growth, affecting ecosystems and agriculture
Even small increases in UV exposure can disrupt delicate biological systems. Marine organisms, particularly those near the surface, are especially vulnerable.
Global Action: The Montreal Protocol
In response to the growing ozone crisis, world leaders signed the Montreal Protocol in 1987 — one of the most successful environmental agreements in history. The treaty phased out the production of major ozone-depleting substances. As a result, atmospheric concentrations of CFCs have steadily declined. Scientists now observe gradual healing of the ozone layer, with projections suggesting significant recovery by the middle of this century. Environmental policy expert Dr. Marcus Lee notes:
“The Montreal Protocol proved that coordinated global action
can reverse even severe environmental damage.”
The ozone hole’s recovery is considered one of humanity’s major environmental success stories.
Current Status and Future Outlook
Although the ozone layer is healing, the Antarctic ozone hole still appears every spring, varying in size depending on temperature and atmospheric conditions. Climate change may influence the speed of recovery, as warming and cooling patterns affect stratospheric chemistry. Continued monitoring and strict regulation of industrial chemicals remain essential to ensure long-term ozone stability. Scientists use satellites and ground-based instruments to track changes, ensuring that global efforts remain effective.
Interesting Facts
- The Antarctic ozone hole was first detected in 1985.
- Some years show ozone thinning over an area larger than 20 million square kilometers.
- A single chlorine atom can destroy thousands of ozone molecules.
- The Montreal Protocol is the only UN treaty ratified by every country in the world.
- If current trends continue, the Antarctic ozone layer may recover to 1980 levels by 2050–2070.
Glossary
- Ozone (O₃) — a molecule in the stratosphere that absorbs harmful UV radiation.
- CFCs (Chlorofluorocarbons) — industrial chemicals once used in refrigeration and aerosols that destroy ozone.
- Polar Stratospheric Clouds — clouds that form in extremely cold conditions and promote ozone-depleting reactions.
- Montreal Protocol — international treaty banning ozone-destroying substances.
- UV Radiation — high-energy sunlight that can damage DNA and living tissues.